Enhanced kits pooling for the quantum shipment model
If your study uses the quantum shipment model, clinical supply managers can now benefit from several enhancements to the existing workflow.
Enhancing the workflow for pooled kits and the quantum shipment model aims to improve the management of material IDs, lots, and shipments. This ensures a more efficient and accurate process, reducing the potential of errors for sponsor users running multiple studies for the same drug compound across multiple vendors.
Additionally, implementing key User Interface (UI) enhancements can benefit any clinical supply manager working with manual shipments, regardless of whether their studies deploy the quantum shipment model or not.
Before you work with this feature
- Create and Edit Library Kits for Pooled Supplies
- Approve Library Kits for Pooled Supplies
- Delete Library Kit
- Create Manual Shipments (Unblinded)
For more information on these global roles and permissions, see Roles for global users and Inventory Management permissions in the Add Users Guide.
Details for clinical supply managers
- When you ship pooled kits through a supply integration, the new Material ID field and the Lot Number value are associated to kits. These values are integrated into the stock report that runs daily and updates the quantities in lots created in your study.
- Each material ID can be associated with multiple countries and multiple kit types, but each unique material ID can only be assigned to one kit type. Each material ID can contain multiple lots, and each lot is assigned to only one material ID. This allows for more precise tracking and management of lots.
- When generating shipments through a resupply strategy (whether it's the first shipment, a manual shipment requested by site, or the resupply shipment created automatically), the system now selects the lowest batch expiry date (first to expire to last to expire) and the lowest material ID (lowest to highest). Moreover, the system ensures that each material ID corresponds to one country and one kit type.
- Resupply shipments are now generated from lots that have a full count of kits. If no lots can ship the full count of kits requested, the resupply shipment cannot be honored and sent to the sites.
- The system now reconciles the count of kits after each shipment request (manual and resupply), ensuring that the inventory is accurately updated.
Figure 2-1 How a clinical supply manager sees the new lot details
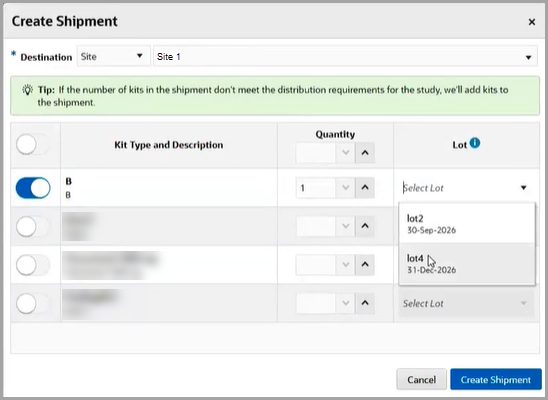
- When you choose to create a manual shipment, on the Create Shipment dialog, the Select Lot drop-down now displays lots specific to every available kit type for serialized, non-serialized, and pooled kits.
- For pooled kits specifically, the Select Lot drop-down only displays lots that correspond to the kit type for the country shipping to.
- On the Select Lot drop-down, you can also see the expiry date for each lot. That way, when you select the lot for a manual shipment, you can make sure the lot won't be expiring before the kits need to be dispensed to the site.
- If the kits included in a shipment pertain to a blinded lot, then the blinded lot expiry date is displayed.
Impact on reports
The new Material ID field is already displayed in the Unblinded Kits dataset in Oracle Clinical One Analytics.
Impact on integrations
If your study uses a SAP integration to ship pooled kits, know that your integration configuration files need to be updated to return the Material ID field during the integration of pooled kit data. For more information, reach out to your Oracle point of contact.
- Depot User Guide
- Sponsor and CRO User Guide
Parent topic: Randomization and trial supply management