Other enhancements
New permission for updating a depot order form
Clinical supply managers are assigned the new Update the Shipment Order Form permission, allowing them to restrict sites' ability to resend the deport order form. This permission is designed to eliminate errors when receiving shipments and fulfilling orders.
Note:
The template study role will be automatically updated. User administrators must update any custom study roles required with this new permission.New notifications on subjects
As a sponsor user, you can now be notified when a subject completes an unscheduled visit, when they are enrolled into a rollover study, and when they complete a study. To receive these notifications, you must be assigned new permissions.
| Permission | Study roles |
|---|---|
| Receive the Subject Completion Notification |
|
| Receive the Subject Rollover Notification |
|
| Receive the Unscheduled Visit Notification |
|
New reports available in Japanese
When you set your language preference as Japanese, the following reports are now available in the Japanese language in all formats:
- Titration Summary report (CSV, HTML, PDF)
- Titration Summary (Unblinded) report (CSV, HTML, PDF)
Keep in mind, clinical data will never be translated and will be reported exactly as collected, regardless of the user's language preference.
New options in Oracle CRF Submit to enhance your work flow
Sponsor and site users can now monitor the progress of an archive request, as well as pause, resume, and cancel requests while they are generating. Also, failed requests can now be resubmitted.
Also, sponsor users can now generate a Custom PDF. The Custom PDF request type gives you access to all settings available in Oracle CRF Submit.
For more information, see Oracle CRF Submit archives and reports in the Oracle Clinical One Platform Reporting Guide.
New integration with SAP system for additional kit identifiers
Sponsor users can now include additional identifiers for kits in the Oracle Clinical One Platform database. These fields are accessible through the SAP system and are populated via an integration.
Although this information will not be available through the Oracle Clinical One Platform interface, kit identifiers will be available to data managers in Oracle Clinical One Analytics as part of an upcoming release.
Introducing the new Redwood design!
The Oracle Clinical One Platform user interface has been refreshed to align better with Redwood, Oracle's new user experience design language. Redwood represents an effort to develop a new Oracle UI to bring natural elements into our products, including Oracle Clinical One Platform. A darker global header with the new Redwood stripe is introduced, as well as sharper fonts and icons.
- System performance.
- The underlying database.
- Data within the application.
- Your ability to perform daily tasks and functions.
- Integrations or external systems.
User interface behavior when the data intake integration populates a future visit
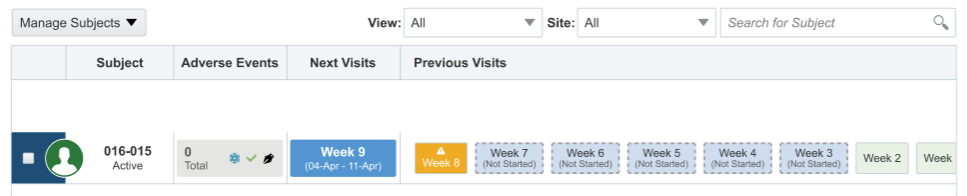
When data is automatically loaded in forms through a data intake integration, the data may be populated in visits out of chronological order as defined in a study's visit schedule. If data is loaded in a future visit, the user interface logically displays any preceding visits available for data entry.
For example, data entry has been completed for visits Week 1 and Week 2 but has not yet been started for visits Week 3 through Week 7. When the data intake integrations, defined for Week 8 and Week 9 are processed:
- Week 3 through Week 7, the preceding, not-yet-started visits are displayed in order, as defined in the visit schedule, have a dotted outline and are labeled (Not Started).
- Week 8 is displayed as the last entry under Previous Visits.
- Week 9 is displayed under Next Visits.
More information will be featured in the Icon Reference Guide, section What statuses can a visit have?.